2022英语周报高考提神版17期综合能力测试13答案


第二节书面表达NoticeDear studentsIn order to carry forward China's time-honoredraditional culture and expand the international influence ofChinese culture. the Student Union of our school will holda Tang and Song Poetry Recitation Contest. All theexchange students who feel interested and have a passionfor Chinese culture are welcome to participate in thecontest. We will appreciate the beauty of Tang and Songpoetry and fecl the charm of Chinese literature togetherLet's recite poetry, taste classics and feel literature. Wewill be seeing each other in the school hall on May 5thThe Student ur
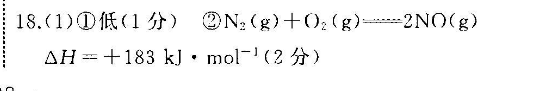


18.(1)①低(1分)②N2(g)+O)2(g)=2NO(g)△H=+183kJ·mol-(2分)(2)①>(1分)②使用催化剂(1分)=(1分)③△H2=2△H1+6△H2[或△H1=(△H2-6△H3)或△H3=(△H2-2△H1)](2分)(2)TiCI (g)+2C0(g)=TiO (s)+2Cl2(g)+2C(s)△H=+45.5kJ·mo1-(2分)②>(1分)【解析】(1)①根据图示可知反应物断键吸收的能量为945kJ+498kJ=1443kJ.生成生成物化学键放出的能量为2×630kJ=1260kJ.吸收的能量大于放出的能量,为吸热反应,则1molO2与1molN2的总能量比2 mol No的总能量低。②反应热为1443kJ·mol-11260kJ·mol-=+183kJ·mol-,则热化学方程式为N2(g)+O2(g)=2NO(g)△H=+183kJ·mol-(2)①通过图中信息可判断反应物总能量小于生成物总能量,属于吸热反应,因此△H1>0。②图中途径Ⅱ的活化能降低,因此条件是使用催化剂,由于催化剂不能改变反应热,则途径I的反应热=途径Ⅱ的反应热。③根据盖斯定律可知(-2×|)×即得到H:(g)+oO(g)H: O(g) AH(△H22△H1)。(3)①利用盖斯定律,将I-Ⅱ,便可得出TCl(g)2CO(g)- TiO,(s)+ 2Ch,(g)+2C(s)AH+45.5kJ·mo1-1。②因为活化能E(正反应)-E(逆反应)=△H,所以若反应Ⅱ的逆反应活化能表示为EkJ·mol1,则E(逆反应)=E(正反应)-△H>220.9。

以上就是2022英语周报高考提神版17期综合能力测试13答案,更多英语周报答案请关注本网站。






